News release
 Top
TopTOKYO, JAPAN - December 1, 2020 - Terumo Corporation (TSE: 4543) today announced that the WEB™ Embolization System (Product name in Japan: Woven EndoBridge Device), an intrasaccular aneurysm treatment device, has launched in Japan. The WEB device is a first-of-its-kind intrasaccular flow disruptor device available for the Japanese market. With the commercial launch, Terumo will provide new treatment options for brain aneurysm cases that were difficult to treat in currently approved methods.
A brain aneurysm is a bulge in the weakened area of a blood vessel in the brain. Patients who endure brain aneurysms are at risk of the aneurysm growing and eventually rupturing, which may lead to severe bleeding in the brain. In order to treat the disease, there are 2 main methods to disrupt excess blood from flowing into the aneurysm. First option is to undergo surgical clipping, where a small metal clip is placed on the neck of the aneurysm. Another option is an intravascular procedure where flow disruption devices such as embolic coils or flow diverter stents are used, passed through by inserting a catheter in the blood vessel.
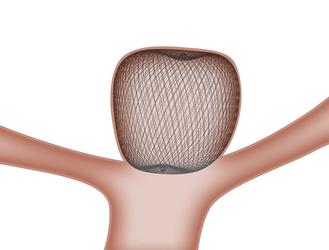
The WEB device is a unique intravascular device that can be used to treat intracranial wide neck bifurcation aneurysms that cannot undergo surgical clipping. Currently, when treating such an aneurysm, it is necessary to use several devices in order to keep the embolic coils inside the aneurysm.
When placed inside the aneurysm sac, the WEB device's proprietary microbraid technology self expands and conforms to the aneurysm shape. It bridges the aneurysm neck, disrupting blood flow, creating a scaffold for long-lasting treatment. The product is a single-device solution, where the total procedure from device insertion to withdrawal is 20.9 minutes on average*.
"The WEB has been CE marked since 2010, PMA approved in the U.S. in 2019, and has been safely used in over 10,000 cases throughout the world. We are excited to provide a new solution for patients in Japan," commented Carsten Schroeder, President and CEO of MicroVention, the U.S. based subsidiary of Terumo and leading global neurovascular company.
- *
Note: 20.9 minutes is the time from WEB system insertion into the micro catheter to system removal and does not imply the total time patients undergo procedure. The figure is based on the cases from the clinical trial for device approval in Japan.
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.