News release
 Top
TopTOKYO, JAPAN and DEVENTER, THE NETHERLANDS - July 15, 2020 - Terumo Corporation (TSE: 4543) today announced it has completed the acquisition of Quirem Medical B.V., a Netherlands-based healthcare startup specializing in the development of next-generation microspheres for Selective Internal Radiation Therapy (SIRT), a treatment for liver tumors. Under the terms of the agreement, Terumo acquired 80.1% of the shares of Quirem Medical. This is over and above its current share position of 19.9%, making Quirem Medical now a wholly owned subsidiary of Terumo.
Terumo will make a one-time, up-front payment of USD 20 million with up to USD 25 million additional payments based on the achievement of future milestones by 2030. It will be funded through cash on hand and will not significantly impact the company's financial projections for the current fiscal year ending March 31, 2021.
Quirem Medical has developed and manufactures QuiremSpheres™, the only commercially available microspheres containing the radioactive isotope Holmium-166. Recent trials have shown the safety and efficacy of holmium microspheres for the treatment of unresectable liver cancer. To improve patient selection, therapy planning and treatment verification, QuiremSpheres can be visualized and quantified even in low concentrations by means of Single-Photon Emission Computed Tomography (SPECT) and Magnetic Resonance Imaging (MRI). This is unique and cannot be done with currently available Yttrium-90 based microspheres.
Furthermore, Quirem Medical also produces QuiremScout™, a low dose holmium microsphere that helps evaluate the biodistribution of microspheres prior to therapy, and a dosimetry software package, Q-Suite™, which is used to plan QuiremSpheres treatments based on QuiremScout dose imaging. Q-Suite is also able to determine SIRT success immediately after the procedure by converting SPECT and MR imaging into absorbed dose distributions. Together, these three integrated products (QuiremSpheres, QuiremScout and Q-Suite) make up the full Holmium SIRT Platform. The Holmium Platform equips physicians with the necessary tools to optimize SIRT outcomes through more personalized treatment, addressing the individual needs of each patient.
QuiremSpheres, QuiremScout and Q-Suite are CE-Marked and currently available in Europe, the Middle East and Africa (EMEA). In the coming years, Terumo intends to launch the Holmium Platform globally as part of the ongoing expansion of its interventional oncology (IO) portfolio.
"The acquisition of Quirem Medical further strengthens our business, expands on our manufacturing and clinical development activities, and complements our comprehensive suite of offerings to support our customers," said Jim Rushworth, Chief Commercial Officer of the Interventional Systems Division of Terumo.
Using Quirem's innovative Holmium-166 platform technology, physicians are further empowered to drive treatment outcomes after SIRT. "By adding the Holmium-166 platform to our existing IO portfolio, we will further contribute to giving liver cancer patients a better future," said Laurent Domas, Vice President, Global Interventional Oncology Strategy & Therapy Development, Terumo Europe. "This acquisition reflects Terumo's commitment to build a broad platform of loco-regional treatment options for liver cancer and is another step forward as we aim to develop and provide treatment solutions for other organs as well."
"We are very excited with the acquisition of Quirem Medical by Terumo as it will further drive the adoption of our unique product offerings worldwide and accelerate our pace of innovation, reaching more patients that will benefit from our technology," said Jan Sigger, CEO of Quirem Medical.
The global interventional oncology market value is more than USD 1 billion, which is a rapidly growing field with a CAGR of 7%. Within this field, SIRT is one of the main treatments that is expected to help to drive this growth year on year.
Terumo has been building its presence in the interventional oncology field, with product offerings such as the micro catheter system (Progreat™), compressible microspheres for embolization (HydroPearl™), drug-eluting microspheres (LifePearl™), and biodegradable drug eluting microspheres (BioPearl™). In 2015, Terumo invested in Quirem Medical and became the exclusive global distributor of their technology.
-
QuiremScout and QuiremSpheres
-
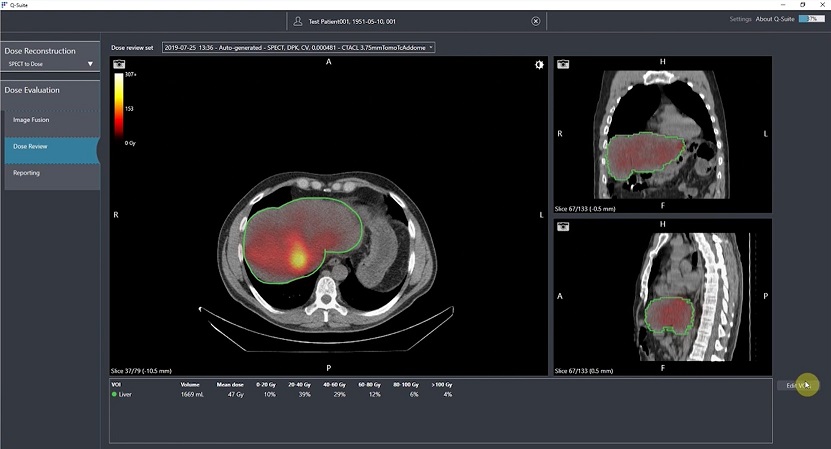
Q-Suite software
About Quirem Medical B.V.
Quirem Medical develops and commercializes the next-generation Selective Internal Radiation Therapy (SIRT) microspheres based on the radioisotope Holmium-166. The company believes the treatment outcome of unresectable liver cancer with SIRT can be optimized with Holmium-166 microspheres, which can be visualized and quantified to improve SIRT patient selection, treatment planning and treatment verification. Its innovative Holmium-166 platform technology provides physicians a complete CE-marked SIRT solution including QuiremScout™, QuiremSpheres™ and the supporting dosimetry software Q-Suite™. The company is based in Deventer, the Netherlands and has approximately 30 employees.
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.
