News release
 Top
TopTOKYO, JAPAN - September 20, 2019 - Terumo Corporation (TSE: 4543) today announced that a new formulation to treat osteoporosis with high risk of fractures, developed by Asahi Kasei Pharma Corporation, has received approval for manufacturing and sales within Japan*. Terumo has signed a manufacturing contract with Asahi Kasei Pharma, and the upcoming product will be manufactured at Terumo Yamaguchi D&D Corporation.
The formulae, TeriboneTM 28.2㎍, is pre-filled in Terumo's PLAJEXTM syringe. Furthermore, the product is assembled as an auto-injector delivery device for a single use dosage. The unique polymer material of PLAJEX TM and Terumo's leading aseptic-filling technology was highly valued when designing this new product.
"With drug and delivery device collaboration, we are able to provide new therapeutic options to patients. We will further contribute to healthcare, by continuing to partner with pharmaceutical companies and cater to their needs", comments Tetsuya Kumei, Division President of the Alliance Division, General Hospital Company of Terumo Corporation.
-

TeriboneTM 28.2㎍ subcutaneous autoinjector
-
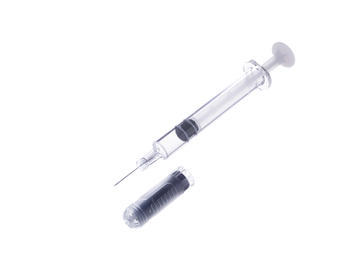
Polymer Pre-Fillable Syringe PLAJEXTM
-
*Asahi Kasei Pharma Press Release on September 20, 2019: "Approval to manufacture and sell Teribone™ autoinjector"
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.
