News release
 Top
TopTOKYO, JAPAN - April 25, 2018 - Terumo Corporation (TSE: 4543) announced today that it acquired CE marking for the Ultimaster™ Tansei™ drug eluting stent (DES). Looking ahead, Terumo will begin sales of the product in Europe in May 2018. On January 24, 2018, Terumo received approval to manufacture and sell Ultimaster Tansei in Japan, and it plans to release the product in the second half of fiscal 2018.
For the Ultimaster Tansei, Terumo inherited the same stent, drug, polymer, and coating methods used for its predecessor, the Ultimaster DES, which was launched in 2014, while making improvements to the tip and shaft in order to facilitate stent delivery in complex lesions. Terumo aimed to enhance the product's usability and make it easier to navigate the stent through the blood vessels by creating a tip with a structure using highly durable yet still flexible materials, and by designing the shaft to have highly kink-resistance, providing exceptional pushability.
Available in a wide range of sizes, Ultimaster Tansei enables a broad variety of treatment options. Terumo will offer a total of 54 size lineup with stent diameter ranging from 2.25 to 4.0 mm, and lengths from 9 to 38 mm in addition to the standard length of 21 mm. Accordingly, the new product will facilitate an extensive range of treatments for complex lesions.
Terumo has been strategically developing the DES under its Mid- to Long-term Growth Strategy. Following the release of the new Ultimaster Tansei, Terumo plans to launch more products with improved stent designs as metal DES in the Ultimaster series, with the goals of securing the number-one share of the DES market in Japan and doubling its market share in Europe and emerging countries. While offering optimal therapeutic alternatives to healthcare professionals, Terumo will work to contribute to improving the quality of life of patients worldwide in the future.
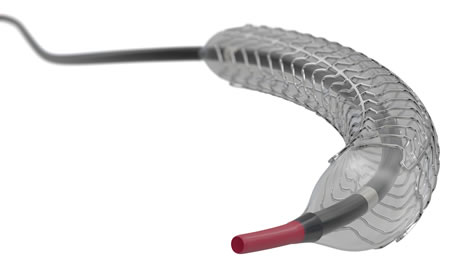
An image of the Ultimaster Tansei, featuring an improved tip and shaft delivery system
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.
