News release
 Top
TopTOKYO, JAPAN - May 23, 2017 - Terumo Corporation (TSE: 4543) announced today that European Medicines Agency (EMA) has validated on May 18 for review the Marketing Authorization Application for a biosimilar to be formulated by Terumo. With this validation, the application is complete and the EMA will now begin the review procedure.
The primary container of the biosimilar is PLAJEX™, Terumo's polymer prefillable syringe. It is the first time that medicines filled in PLAJEX™ have been applied outside Japan.
Based on this achievement, Terumo will continue to expand its alliance business with pharmaceutical companies by taking advantage of material technology and aseptic filling technology of prefillable syringes.
About PLAJEX™
PLAJEX™ is a sterile ready-to-fill polymer syringe developed by Terumo with a strong focus towards biopharmaceuticals. Key features of PLAJEX™ include:
- a silicone oil-free prefilled syringe system for mitigating protein aggregation, integrating Terumo's proprietary i-coating™ plunger stopper*1.
- steam sterilized for mitigating protein oxidation and aggregation*2.
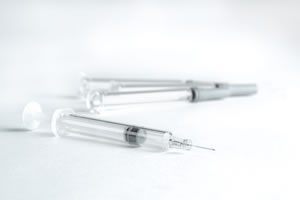
Ready-to-Fill Polymer Syringe PLAJEX™

Tub and Nest
You can learn more about the PLAJEX™ at: http://www.terumo-europe.com/en-emea/pharmaceutical-solutions/syringes/pre-fillable-syringe-systems-cop
About the biosimilar
The biosimilar is FKB327, an adalimumab biosimilar candidate of the fully human anti-TNF-α monoclonal antibody referencing Humira®. In October, 2016, FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd. announced positive result in primary endpoint from Phase 3 global clinical study of FKB327.
Terumo has developed the biosimilar as a combination product that combines drug and device, collaborating with Fujifilm Kyowa Kirin Biologics and others. They adopted Terumo as a contract manufacturing organization.
About FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd.
FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd. was established by FUJIFILM Corporation and Kyowa Hakko Kirin Co., Ltd. on March 27, 2012 as a company for developing, manufacturing, and marketing biosimilars.
- ※1
Silicone oil has been used as lubrication to achieve smooth plunger gliding functionality. However, in the context of biomolecules, silicone oil became a serious issue because it can induce protein aggregation. Terumo has developed a silicone oil-free prefillable polymer syringe system based on a plunger stopper combined with a specific coating technology, called i-coating™ providing smooth surface layer and consistent gliding forces over time.
- ※2
Earlier publications indicated that protein oxidation was induced in polymer prefillable syringes generated radicals by irradiation sterilization. On the other hand, no remarkable protein oxidation or radical generation was observed in steam-sterilized polymer prefillable syringe. Using steam sterilization as the sterilization method of PLAJEX™ may assist in mitigating interactions with the biopharmaceuticals by reducing the risks of protein oxidation.
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.
