Top
Top(Caution) There are currently no medical devices approved for the specific indication of treating COVID-19 in Japan. The initiatives introduced in this article are example cases being tested at some medical institutions outside of Japan. Therefore, it is not our intention to recommend the use of our products or technologies. Please refrain from contacting medical institutions and government agencies to inquire about the medical devices and technologies appearing in this article.
One of Terumo’s three companies, US-based Terumo Blood and Cell Technologies is actively working to utilize blood and cell technologies to help in the fight against COVID-19.
Centrifugal Apheresis Technology—the Technologies to Receive
Emergency Use Authorization*1 (EUA) from the FDA
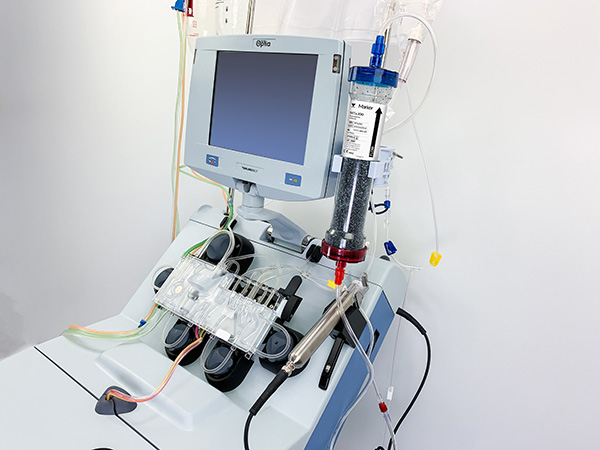
In April 2020, the combination of Terumo Blood and Cell Technologies’ centrifugal apheresis system and the adsorption cartridge of partner company Marker Therapeutics (Switzerland) received Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) for treating COVID-19.
The conditions of the authorization were that the treatment to be used for patients 18 years of age or older who are admitted to the intensive care unit (ICU) with respiratory failure due to COVID-19, and it is granted for the duration of a public health emergency only.
Patients with COVID-19 may suffer from an excessive immune response (cytokine storm) accompanied by severe respiratory disturbances caused by cytokines, a type of protein. To prevent this, the patient's blood is separated into blood cells and plasma through centrifugation, and the cytokines in the plasma are further reduced and returned to the patient's body. Two companies' technologies are being used for this process.
FDA approval for this new treatment took only four weeks from the time that it was submitted for consideration. This unprecedented speed of the approval was made possible by Terumo Blood and Cell Technologies’ accumulation of years’ worth of research data on technologies that separate specific cells within the blood. At the same time, it was also thanks to the quick sharing of information with the FDA under time limitations and support from medical device industry associations. We will aim to contribute to the treatment of even more patients going forward.
- *1
About Emergency Use Authorization Status
Component Collection System—Bringing Plasma
from Recovered Patients to Research Sites
No effective medical treatment for COVID-19 itself has been discovered yet, and research efforts are proceeding at breakneck pace around the globe to find an effective treatment as quickly as possible. Amongst these efforts, one treatment method in the US that has been gaining attention uses “convalescent plasma” with virus antibodies in blood taken from patients who have recovered from COVID-19. Together with the American Red Cross, the FDA is calling on people across the US who have recovered from COVID-19 to donate their plasma while also providing directions to local blood centers on their website. Terumo Blood and Cell Technologies component collection system is playing an active role at many blood plasma collection sites. Convalescent plasma is currently undergoing clinical trials, as a treatment method for COVID-19, and research to validate its safety and effectiveness is ongoing.
Terumo Blood and Cell Technologies will continue to keep a close watch on the outcomes of this treatment while supporting research sites by providing proper information and training to medical professionals and otherwise contributing in any way possible.
The Spectra Optia Apheresis System with the D2000 Adsorption Cartridge is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of the Spectra Optia Apheresis System with the D2000 Adsorption Cartridge under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Currently, there are no FDA-approved, licensed, or cleared device treatments for COVID-19.