News release
 Top
TopTOKYO, JAPAN - November 9, 2020 - Terumo Corporation (TSE: 4543), a global leader in medical technology, today announced that it has received CE Mark for the MEDISAFE WITH™ insulin pump system. MEDISAFE WITH is developed and manufactured in Japan; and it is the first and only tubing-free patch pump for insulin delivery available in Japan since its commercial launch in 2018. Having obtained the CE mark, Terumo will initiate the global product launch.
"We are thrilled to share this positive news to the world. At Terumo, one of our Core Values is 'Care - Empathetic to patients' and when we designed MEDISAFE WITH, 'patient friendly' was our first priority. With the wearable lightweight detachable pump, we hope to free patients from the stress they face every day, such as finding a location to inject insulin, or having limited options on clothing due to the device," commented Hikaru Samejima, President of Terumo's General Hospital Company. "We promise to strive to give patients a better life."
MEDISAFE WITH is designed to be wearable and lightweight*1 for patient comfort and freedom. The system is handy sized*2, consisting of two parts including a detachable pump and patch. Insulin is administered subcutaneously through a cannula attached to the patch. Administration settings such as basal and bolus can be adjusted using a remote controller when needed.

Patients can administer insulin on the spot with the MEDISAFE WITH
About Insulin Pump Therapy (CSII: Continuous Subcutaneous Insulin Infusion)
Type 1 diabetes is caused by an autoimmune reaction in which the body's immune system attacks the insulin-producing beta cells of the pancreas. As a result, the body produces very little or no insulin. Currently there are over 1,100,000 Type 1 diabetes patients under the age of 20 and this number is increasing year by year.*3
To maintain appropriate glucose levels, patients with Type 1 diabetes need daily insulin injections, usually up to 4 times a day. Chronically high level of glucose may lead to complications such as retinopathy and nephropathy.
Insulin pump therapy is a treatment in which insulin is continuously administered using a portable pump. The insulin is delivered into the patient's body through a subcutaneously inserted cannula. Because the insulin dosage can be adjusted accurately depending on day-to-day activities such as exercise and meal choice, it is expected to improve glucose management, and to help preventing complications*4, *5.
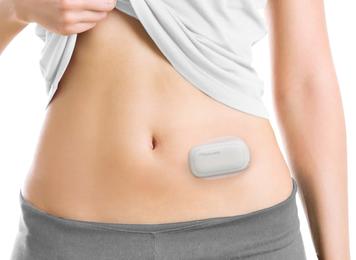
Patient wearing MEDISAFE WITH

(Left) Remote controller
(Right) Pump
- *1
34g (weight when combined with main pump unit, cartridge and patch, not including insulin)
- *2
77.9mm (L) x 40.1mm (D) x 18.9mm (W) (dimensions when combined with main pump unit, cartridge and patch, not including cannula length)
- *3
International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation, 2019.
- *4
Isabelle, Steineck. "Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18168 people with type 1diabetes: observational study" BMJ,2015;350:h3234
https://www.bmj.com/content/350/bmj.h3234 - *5
John, C. Pickup. "Management of diabetes mellitus: is the pump mightier than the pen?" Nat. Rev. Endocrinol. 8, 425-433 (2012);
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.
