Top
TopA development project of passion and challenge,
a thirty-year pursuit of excellence
Heart transplant is the last option for the treatment of patients with severe heart failure whose heart disease has worsened to the point of heart impairment. There are many patients who need a transplant but must wait for a suitable donor to be found. An Implantable Left Ventricular Assist System is a medical device that sustains life by assisting the patient’s heart while a patient waits for a transplant. Terumo developed its product through much trial and error, aiming to reduce physical burdens on patients as much as possible, and with the belief that patients should be enabled to lead and enjoy their normal lives.
This project to develop Terumo's Implantable Left Ventricular Assist System is about to end its 30-year history, having already completed the new product development and now only providing support to ongoing patients who are using the product.
However, the Implantable Left Ventricular Assist System is a product of great significance to Terumo. While it is important that the new technologies and knowledge gained during its development have been passed on to subsequent research and development, it is no exaggeration to say that this is one of the products that Terumo associates have felt most closely about their corporate mission of "Contributing to Society through Healthcare". In this article, we will look back at the history of this product.
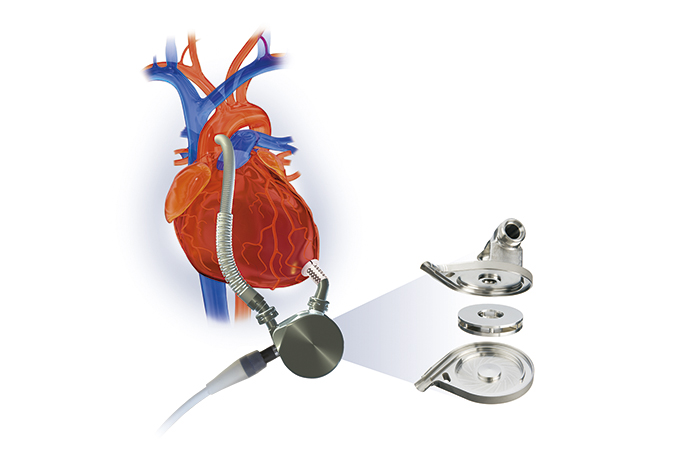
Continual evolution of the artificial heart
To save patients with severe heart failure
The development of artificial hearts to treat patients with severe heart failure began in the 1930s, and at that time, the goal was to mimic the actual heart. The treatment back then consisted of removing the heart and replacing its function with a device. However, the early artificial hearts had many problems: Durability was poor, and patients were exposed to the risk of blood clots and infections, and the large size of the device caused the patients to become bedridden.
After years of difficulties to put the artificial heart to practical use, the development of the artificial heart reached a turning point in 1969. The role of the artificial heart changed drastically when a new concept was born, from "replacing the heart" to "using it as a bridge" to a heart transplant. Furthermore, since the left ventricle was the most demanding and vulnerable part of the patient's heart function, the left ventricular assist device, which helps the left ventricle function and promotes the recovery of heart function, became the mainstream of development. In 1981, Dr. Tetsuzo Akutsu, a cardiac surgeon, created an artificial heart that was used as a "bridge" to a heart transplant, and the era of full-fledged clinical use began.
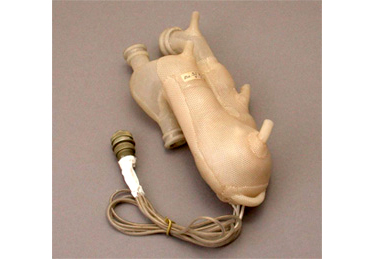
Early artificial heart created by Dr. Akutsu
Total Artificial Heart (TAH)
Akutsu Model III
(From Division of Surgery: Texas Heart Institute, 8(3), 305-319,1981)
The artificial heart development at Terumo began in 1983 in a joint effort to develop a pump with Hokkaido University. However, that project was paused due to technical issues. In 1989, by the invitation of Terumo’s then-President Mitsuo Tozawa, Dr. Akutsu, a leading expert in the development of artificial heart, became Vice President and Chief of Artificial Heart Development at Terumo. Further, in 1991, Terumo invited Dr. Chisato Nojiri, a cardiac surgeon at Tokyo Women's Medical University, to become a visiting researcher at its research and development center, and Terumo’s development of the heart assist system restarted in earnest.
Pursuit to protect blood -
Birth of the third-generation
magnetic levitation centrifugal pump
The development of the artificial heart pump technology evolved from the first-generation pulsatile-flow pump, which pumped blood in a rhythm similar to the natural heart, to the second-generation continuous-flow pump, which pumped blood in a constant flow like a water pump. Systems using this second-generation pump became smaller and lighter, significantly reducing the burden on patients compared to early artificial hearts. However, there were problems such as friction between the shaft that rotates the impeller that pumps the blood and its bearing, resulting in rotation issues, and blood coming into contact with foreign objects leading to blood clots.
To solve this biggest challenge in the development of ventricular assist devices, which is how to prevent blood clots, Terumo decided in 1994 to introduce a technology called "magnetic levitation centrifugal pump", which was invented by Kyoto University and bearing manufacturer NTN Corporation, and began a joint research project to that end. This technology uses magnetic force to rotate the impeller while keeping it afloat. The idea was if the mechanical friction in the blood chamber is eliminated, the risk of blood clots should be reduced. In 1997, a ventricular assist device using this pump technology set a world record (at that time) by operating for 864 consecutive days in animal testing, confirming that the technology could enable a long-term use of the pump. In addition, the material for the main pump was upgraded to titanium, and in 1998, the decision was made to commercialize the Implantable Left Ventricular Assist System.
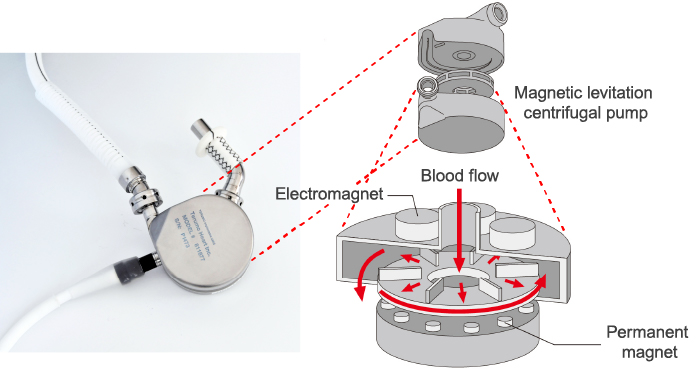
Magnetic levitation centrifugal pump structure
Overcoming post-market technical challenges however, the project comes to an end under difficult market conditions
At the time, Terumo had never commercialized a Class IV medical device—the class of medical device with highest risk to the human body—and the environment for developing such product was not yet ready in Japan.
In the United States, however, national policy had promoted the advancement of the medical device industry, creating an environment that offers the technologies and talent needed for the product development of this magnitude. To bring the product to patients as soon as possible, Terumo therefore moved its development base to the United States in 2000.
Clinical trials of Terumo’s Implantable Left Ventricular Assist System began in Europe in January 2004, followed by the United States and Japan. Faced with a variety of challenges, clinical trials continued, and finally the implantable left ventricular assist device system was launched in Europe in 2007 and in Japan in 2011. However, due to a problem that the pump could not maintain its normal rotation mode (magnetic levitation mode), new implantations were temporarily suspended in Japan in the same year.
In July 2013, sales were resumed after a series of improvements to the device. However, the limitations related to the reliable procurement of electronic components, product supply and equipment maintenance put restrictions on how long the product could be supported. Due to these limitations, Terumo announced to medical institutions that the product would no longer be sold for new implantations as of the end of March 2017. In Europe and the U.S. as well, Terumo faced stiff competition from competitors, and in 2012 Terumo decided to end sales in Europe and suspend clinical trials in the U.S. Although the development of the next-generation device, which was even smaller than the first generation, was underway, the technology was sold to another company in 2013, and the development activities came to an end.
There are currently (as of March 2021) two patients implanted with Terumo’s Implantable Left Ventricular Assist System. Terumo will continue to provide the same support to patients until the day they no longer need the product.
Pioneering in the development of ventricular assist system, contributing to the improvement of healthcare quality
The healthcare environment surrounding implantable ventricular assist systems has changed dramatically in the past decade. In Japan, the number of medical facilities that have been allowed to implant ventricular assist systems has increased from only five in the beginning to 46 today. In addition, the number of models approved for use has increased to five, including products from overseas. The use of assist systems, which was once rare, is gradually becoming to be the standard treatment. Although product development of this device has come to an end at Terumo, the company is proud of its contributions to improving medical technologies and spreading life-saving treatments.
In addition, the various technologies created by the project team over the course of development will continue to live on in future products. Dr. Nojiri, who was the pillar of the development project, passed away on November 13, 2015. Her presence was enormous, and everyone on the project team shared her desire "to help patients in need." With that conviction, this landmark project lasted for 30 years brought into reality the Terumo’s vision of that time, which was “Realize patient-friendly healthcare through unique and brilliant technologies”. The many years the team spent on the project became a priceless asset. These experiences and strong beliefs have been passed down as Terumo’s DNA.