News release
 Top
TopTOKYO, JAPAN - January 8, 2020 - Terumo Corporation (TSE: 4543), a global leader in medical technology, today announced that it has received manufacturing and marketing approval of its Woven EndoBridge Device (WEB™) for the treatment of intracranial wide neck bifurcation aneurysms in Japan. The WEB is the first approved device in Japan as an intrasaccular flow disruptor for aneurysm embolization. After its insurance coverage, Terumo will launch the product in the fiscal year ending March 2021.
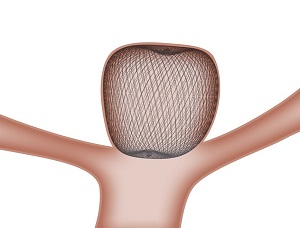
The WEB is a unique, single-device treatment solution for wide neck bifurcation aneurysms. When placed inside the aneurysm sac, the WEB device's proprietary microbraid technology bridges the aneurysm neck, disrupting blood flow, and creates a scaffold for long-lasting treatment. The product is expected to provide a new solution for intracranial wide neck bifurcation aneurysms, which are difficult to treat with conventional devices.
The WEB has been CE marked since 2010 and PMA approved since 2019, and has been safely used in approximately 10,000 cases and multiple clinical studies throughout the world.
Terumo holds "Empathetic to patients" and "Striving for innovation" in its Core Values. The Company strives to give patients a better future by delivering products that create meaningful value to medical settings.
- *
The WEB was developed by Sequent Medical, Inc. and Sequent was acquired by Terumo in 2016. Now MicroVention, Inc., a U.S.-based subsidiary of Terumo and a global neurovascular company, integrated and administers Sequent's functions. W-EB is a pet name in Japan. WEB is a registered trademark in U.S.
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.