News release
 Top
TopMarch 4, 2016 - Terumo Corporation announces today that its MEDISAFE Fit Smile blood glucose meter has received an iF Design Award, an internationally respected award for excellence in design.
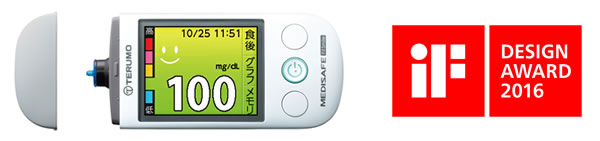
The MEDISAFE Fit Smile is designed to make it easy to understand blood glucose levels and features an attractive shape that users can appreciate. As such, it was recognized by the award from among many other blood glucose meters, which are primarily designed to ensure measurement precision, for a user-friendly design that encourages diabetes patients to continue treatment. Terumo places importance on designing products that people around the world will love, and remains dedicated to the development of medical instruments that can be easily used by both healthcare providers and medical patients in the future.
The winning product page in iF DESIGN AWARD:
About the MEDISAFE Fit Smile
The MEDISAFE Fit Smile is a blood glucose meter used by diabetes patients for managing blood glucose levels. Since Terumo began selling blood glucose meters in 1993, it has striven to supply products that not only perform well but also offer new value. For instance, it began using three-dimensional tips for its lineup of blood glucose meters sold on the market in 1997, an innovation that allowed countless people to use the meters more easily.
The MEDISAFE Fit Smile, Terumo's latest model, has been designed with various functions for helping patients everywhere manage diabetes, including the following.
- Simple operations, including powering on and off when the cap is opened or closed
- Instructions are given on the display and via a built-in voice function
- Five levels of blood glucose are indicated by corresponding background colors on the display
Since launching the product in Japan in October 2014 under the brand name of MEDISAFE Fit Smile, Terumo has expanded sales to countries in Southeast Asia, commenced sales in Italy in January 2016 and will launch in Germany in April 2016.
About the iF Design Award
Awarded annually since 1953 by the iF International Forum Design, a German-based organization, the iF Design Award has become an internationally respected award for recognizing quality design. Each year, entries are evaluated by an expert panel of judges, which includes renowned designers and personnel in charge of design at major corporations. Outstanding entries are selected for the award based on various criteria, including innovativeness, beauty, ease of use, as well as economy and environmental friendliness. In 2016, 1,821 products were awarded from among 5,295 entries from 53 countries worldwide.
About Terumo
Terumo (TSE: 4543) is a global leader in medical technology and has been committed to “Contributing to Society through Healthcare” for 100 years. Based in Tokyo and operating globally, Terumo employs more than 30,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice such as transfusion systems, diabetes care, and peritoneal dialysis treatments. Terumo will further strive to be of value to patients, medical professionals, and society at large.
